We have previously investigated the plant-fungal interaction system of Lotus japonicus (model legume) with an endophytic Fusarium solani (strain K, FsK). Based on our findings, the endophyte colonizes the root system of L. japonicus, and progresses towards the vasculature bundle. Sectioning of the stem close to the root crown has also shown fungal structures of round shape and of unknown function close to xylem cells.
The endophyte, in its association with L. japonicus, employs gene members of the chitin signaling (perception of chitooligosaccharides via LysM receptors at the root epidermis) and the Common Symbiotic Signaling Pathway (the single molecular pathway employed by true symbionts – Arbuscular Mycorrhizal Fungi and Rhizobia – to colonize host plant tissues).
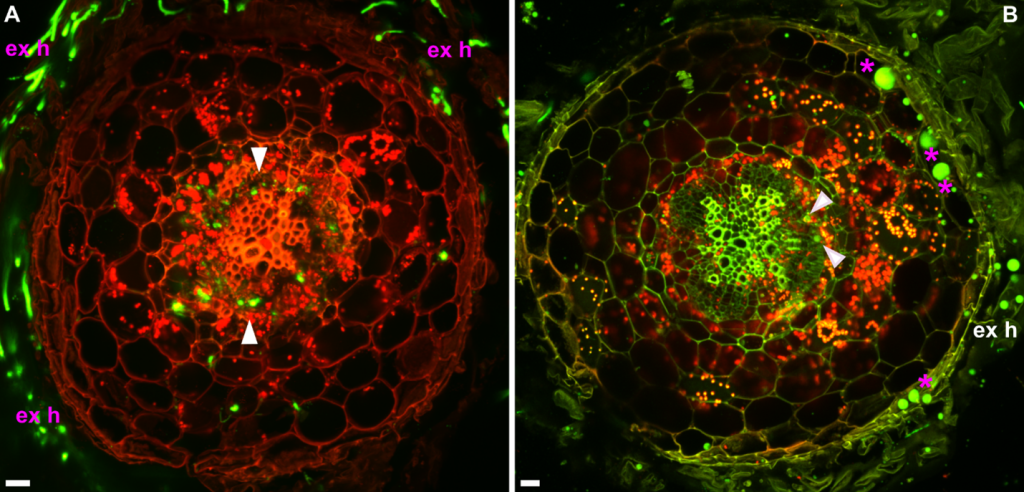
Figure 1 (A) Cross section of the root at a site close to root crown. Fluorescence is detected in the vascular system of the root (arrows). Extraradical mycelium is also recorded. (B) Cross section of the stem, at a site very close to the root crown. Fungal structures of round shape are formed in epidermal/upper cortical cells (asterisks). Green fluorescence close to xylem cells, presumably of fungal origin is also observed (arrows). Confocal microscope images are z axis projections of serial optical sections.single arrow, intraradical colonization; ex h, extraradical hyphae and hyphae on the space surrounding the stem; magenta asterisk, round shaped fungal structures. Bars=20 μm. Images taken by Vasiliki Skiada.
The mode of progression of FsK within L. japonicus root tissues necessitates further investigation, and this is the goal of ‘EndoPath’.
Plant cell wall alterations (structural and chemical modifications), occurring upon root tissues colonization by the endophyte have not been studied to date. Several genes involved in ‘cell wall synthesis and modifications’ as well as ‘cell modifications’ have been identified to be regulated in FsK-Lotus system, via RNA sequencing analysis (Skiada, PhD thesis, 2019). In addition, the potential role of root apoplastic barriers such as endodermis (and its major components lignin, and suberin) in allowing/restricting fungal progression is not yet clear in our plant-fungal interaction system.
Last but not least, the potential integration of chitin and/or symbiotic signaling pathway with plant cell/cell wall modification responses upon FsK colonization is also not known.
- Kavroulakis N, Ntougias S, Zervakis GI, Ehaliotis C, Haralampidis K, Papadopoulou KK. (2007), Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. Journal of Experimental Botany 58: 3853–3864. https://doi.org/10.1093/jxb/erm230
- Skiada V., Faccio A., Kavroulakis N., Genre A., Bonfante P., Papadopoulou KK. (2019), Colonization of legumes by an endophytic Fusarium solani strain FsK reveals common features to symbionts or pathogens,Fungal Genetics and Biology, 127: 60-74, https://doi.org/10.1016/j.fgb.2019.03.003.
- Skiada, V., Avramidou, M., Bonfante, P., Genre, A. and Papadopoulou, K.K. (2020), An endophytic Fusarium–legume association is partially dependent on the common symbiotic signalling pathway. New Phytol, 226: 1429-1444. https://doi.org/10.1111/nph.16457
- Tsiknia M., Tsikou D., Papadopoulou K.K., and Ehaliotis C. (2021), Multi-species relationships in legume roots: From pairwise legume-symbiont interactions to the plant – microbiome – soil continuum. FEMS Microbiology Ecology, 97(2), fiaa222. https://doi.org/10.1093/femsec/fiaa222
‘EndoPath’ aims to answer the following questions:
- What are the cellular and molecular mechanisms related to plant cell wall alteration/fortification and the role of endodermis in endophytic filamentous fungi progression in legume plants?
- Are there any commonalities in these mechanisms in pathogenic/symbiotic fungal progression?
- How do fungi employ these mechanisms at the tissue level to ensure their progression towards inner root tissues?
- Is there a correlation between chitin/symbiotic signaling pathways and pathways related to plant cell wall reinforcement/loosening?
- How do these processes reflect on changes in chemical composition of plant tissues inoculated with interacting fungi?

1. In silico approaches:
mining of genes from previously gained RNA sequencing data; mining of genes from public databases; transcript sequences identification; primer design

2. Molecular biology tools:
targeted gene expression analyses; promoter activity experiments, to study gene expression of selected genes in infected plant tissues

3. Bright-field and Advanced microscopy tools:
both bright-field and fluorescence microscopy (confocal microscopy) will be used to investigate: plant fortification substances deposition upon colonization, spatiotemporal gene expression of genes of interest in infected plant tissues.

4. Chemical analysis tools:
extraction of plant cell walls substances of interest from control and fungi-infected plant tissues; analysis via phasmatophotometric approaches and/or other analytic methods
images from pixabay.com
A brief presentation of ‘EndoPath’ work packages (WPs) is described below:
Previously acquired RNA sequencing data will be screened to identify genes of interest.
Genes from publicly available data also reported to be involved in similar processes will be investigated.
Plants will be screened for alterations in fortification substances deposition in the presence of selected fungi.
Gene expression of selected genes will be investigated in infected plant tissues by employing promoter analysis experiments.
Deposition/fortification substances will be extracted from control and infected with the selected fungi plant tissues and quantified to record differences by employing analytical approaches.
A range of communication and dissemination tools (website creation, communication via social media etc.) will be used throughout the project duration, to ensure that goals and objectives are effectively communicated to the public.